Abstract: The endocannabinoid system (ECS) is a ubiquitous and vital regulatory system involved in maintaining homeostasis throughout the body. Dysfunction within this system has been implicated in a growing range of illnesses, spanning neurological, psychiatric, immune, and metabolic disorders. Cannabinoid therapy, utilizing exogenous cannabinoids like phytocannabinoids from the cannabis plant and synthetic analogs, offers a promising avenue for modulating the ECS and alleviating symptoms associated with these conditions. This paper explores the ECS's role in various illnesses and examines the potential therapeutic applications of cannabinoid therapy.
1. Introduction:
The discovery of the endocannabinoid system (ECS) in the late 20th century revolutionized our understanding of human physiology. The ECS is composed of cannabinoid receptors (primarily CB1 and CB2), endogenous ligands known as endocannabinoids (eCBs) like anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and the enzymes responsible for their synthesis and degradation. This intricate network plays a critical role in regulating various physiological processes, including pain perception, inflammation, appetite, mood, memory, and immune function. Dysregulation of the ECS can contribute to the pathogenesis of numerous diseases, making it a compelling therapeutic target.
2. The Endocannabinoid System: A Primer:
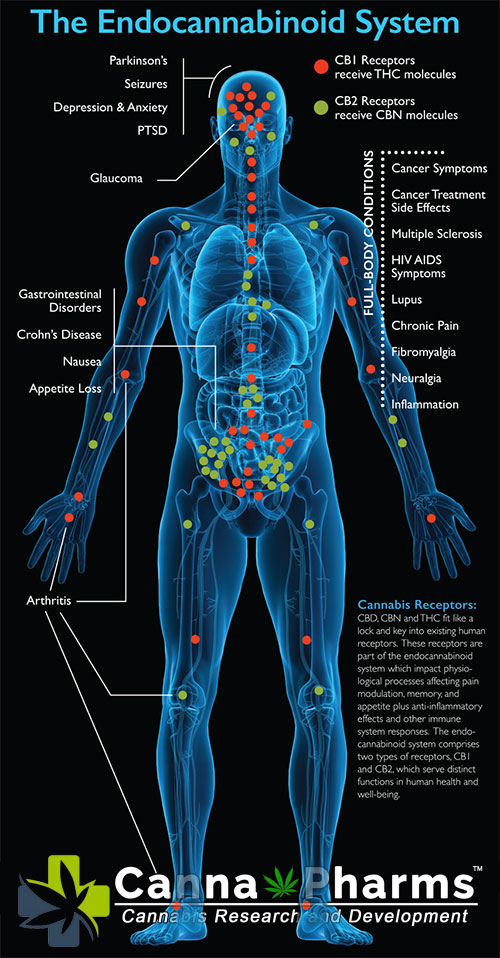
The ECS is a dynamic and complex system operating in a retrograde manner. When a neuron is overstimulated, it releases eCBs which then travel backward across the synapse to activate cannabinoid receptors on the presynaptic neuron. This activation leads to a reduction in neurotransmitter release, effectively dampening neuronal activity and restoring balance. CB1 receptors are predominantly found in the brain and central nervous system, whereas CB2 receptors are primarily located on immune cells and peripheral tissues. However, it's crucial to note that this distribution is not absolute, and both receptor types can be found in various tissues.
Endocannabinoids, synthesized "on-demand" from membrane lipids, are rapidly broken down by enzymes like fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL). This rapid turnover ensures precise and localized control of ECS signaling. Factors such as genetics, lifestyle, diet, and environmental exposures can significantly influence the function of the ECS, potentially contributing to disease development.
3. ECS Dysregulation in Disease and the Promise of Cannabinoid Therapy:
The involvement of the ECS in a wide range of physiological processes highlights its potential implication in various disease states. Cannabinoid therapy aims to modulate the ECS by utilizing exogenous cannabinoids that interact with CB1 and CB2 receptors, as well as influencing eCB levels by inhibiting their degradation enzymes.
3.1. Neurological Disorders:
- Multiple Sclerosis (MS): Studies have shown that activation of cannabinoid receptors can reduce spasticity, pain, and bladder dysfunction in MS patients. Nabiximols (Sativex), a THC/CBD oromucosal spray, is approved in many countries for the treatment of MS-related spasticity.
- Epilepsy: CBD has demonstrated anticonvulsant properties in several clinical trials. Epidiolex, a purified CBD formulation, is approved by the FDA for the treatment of specific types of epilepsy, including Dravet syndrome and Lennox-Gastaut syndrome. The exact mechanism of action is still under investigation, but it is believed to involve modulation of neuronal excitability and interactions with other receptors.
- Neurodegenerative Diseases (Alzheimer's and Parkinson's): While more research is needed, preclinical studies suggest that cannabinoids may offer neuroprotective effects by reducing inflammation, oxidative stress, and amyloid-beta aggregation in Alzheimer's disease. In Parkinson's disease, cannabinoids may alleviate motor symptoms like tremors and rigidity.
- Chronic Pain: The ECS plays a critical role in pain modulation. Cannabinoids, particularly THC, can activate CB1 receptors in the brain and spinal cord, reducing pain perception. Cannabinoid therapy has shown promise in managing chronic pain conditions such as neuropathic pain, fibromyalgia, and arthritis.
3.2. Psychiatric Disorders:
- Anxiety and Depression: The ECS is implicated in mood regulation and stress response. Low doses of THC may have anxiolytic effects, while higher doses can increase anxiety. CBD has shown potential as an anxiolytic and antidepressant, although the optimal dosage and long-term effects require further investigation. The potential for therapeutic benefits must be carefully weighed against potential risks, particularly in individuals with a history of psychiatric disorders.
- Post-Traumatic Stress Disorder (PTSD): The ECS is involved in the extinction of fear memories, a process impaired in PTSD. Cannabinoids may facilitate the forgetting of traumatic memories and reduce anxiety associated with PTSD symptoms. However, research in this area is still in its early stages.
- Schizophrenia: While THC can exacerbate psychotic symptoms in vulnerable individuals, CBD has shown potential as an antipsychotic agent. Research suggests that CBD may modulate dopamine neurotransmission and improve cognitive function in schizophrenia.
3.3. Immune Disorders:
- Inflammatory Bowel Disease (IBD): The ECS plays a regulatory role in intestinal inflammation. Cannabinoids can reduce inflammation, pain, and intestinal permeability in IBD models. Clinical studies exploring the efficacy of cannabinoid therapy in IBD are ongoing.
- Arthritis: CB2 receptor activation has been shown to reduce inflammation and pain associated with arthritis. Topical application of cannabinoid-containing creams may offer localized pain relief.
- Autoimmune Diseases: By modulating immune cell activity, cannabinoids may have therapeutic potential in autoimmune diseases such as rheumatoid arthritis and lupus.
3.4. Metabolic Disorders:
- Obesity and Diabetes: The ECS influences appetite, energy expenditure, and glucose metabolism. CB1 receptor antagonists were initially developed as anti-obesity drugs but were withdrawn from the market due to psychiatric side effects. However, research continues to explore the potential of targeting the ECS in metabolic disorders, with a focus on selective CB2 receptor agonists and compounds that modulate eCB levels.
4. Challenges and Future Directions:
Despite the promising therapeutic potential of cannabinoid therapy, several challenges need to be addressed:
- Variability in Cannabis Products: The lack of standardized formulations and quality control in the cannabis industry can lead to inconsistent product potency and purity, making it difficult to determine optimal dosages and ensure patient safety.
- Psychotropic Effects of THC: The psychoactive effects of THC can be undesirable for some patients. Research is focused on developing cannabinoid-based therapies with minimal psychotropic effects, such as CBD and selective CB2 receptor agonists.
- Long-Term Effects and Safety: More research is needed to assess the long-term effects and safety of cannabinoid therapy, particularly in vulnerable populations such as adolescents and pregnant women.
- Drug Interactions: Cannabinoids can interact with other medications, potentially affecting their efficacy or increasing the risk of side effects. Healthcare providers need to be aware of these potential interactions when prescribing cannabinoid therapy.
- Regulatory Hurdles: The legal status of cannabis varies widely across countries and regions, hindering research and access to cannabinoid-based therapies.
Future research should focus on:
- Identifying specific ECS targets for different diseases: Understanding the precise mechanisms by which the ECS contributes to disease pathogenesis will allow for the development of more targeted and effective therapies.
- Developing novel cannabinoids and endocannabinoid modulators: Researchers are exploring new synthetic cannabinoids, selective CB2 receptor agonists, and compounds that modulate eCB levels, aiming to maximize therapeutic benefits while minimizing side effects.
- Conducting rigorous clinical trials: Well-designed clinical trials are needed to evaluate the efficacy and safety of cannabinoid therapy for various conditions.
- Personalized medicine approaches: Factors such as genetics, lifestyle, and disease severity can influence the response to cannabinoid therapy. Personalized medicine approaches may help to identify patients who are most likely to benefit from this treatment.
5. Conclusion:
The endocannabinoid system represents a crucial regulatory system with implications for a vast number of illnesses. Cannabinoid therapy offers a promising avenue for modulating the ECS and alleviating symptoms associated with neurological, psychiatric, immune, and metabolic disorders. While challenges remain, ongoing research is expanding our understanding of the ECS and paving the way for the development of safer and more effective cannabinoid-based therapies. A deeper understanding of the ECS and its role in health and disease is essential to unlock the full therapeutic potential of cannabinoid therapy and improve the lives of patients suffering from a wide range of conditions.
References:
- Battista N., Di Tommaso M., Bari M., Maccarrone M. The endocannabinoid system: An overview. Front. Behav. Neurosci. 2012;6:9. doi: 10.3389/fnbeh.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizpurua-Olaizola O., Elezgarai I., Rico-Barrio I., Zarandona I., Etxebarria N., Usobiaga A. Targeting the endocannabinoid system: Future therapeutic strategies. Drug Discov. Today. 2017;22:105–110. doi: 10.1016/j.drudis.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Lowe H., Toyang N., Steele B., Bryant J., Ngwa W. The endocannabinoid system: A potential target for the treatment of various diseases. Int. J. Mol. Sci. 2021;22:9472. doi: 10.3390/ijms22179472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Śledziński P., Nowak-Terpiłowska A., Zeyland J. Cannabinoids in medicine: Cancer, immunity, and microbial diseases. Int. J. Mol. Sci. 2020;22:263. doi: 10.3390/ijms22010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Walker JM, Huang SM. Cannabinoid analgesia. Pharmacol Ther. 2002;95:127–35. doi: 10.1016/s0163-7258(02)00252-8. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7:438–55. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8:403–21. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos Trans R Soc Lond B Biol Sci. 2012;367:3353–63. doi: 10.1098/rstb.2011.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulugol A, Ozyigit F, Yesilyurt O, Dogrul A. The additive antinociceptive interaction between WIN 55,212-2, a cannabinoid agonist, and ketorolac. Anesth Analg. 2006;102:443–7. doi: 10.1213/01.ane.0000194587.94260.1d. [DOI] [PubMed] [Google Scholar]
- Gunduz O, Karadag HC, Ulugol A. Synergistic anti-allodynic effects of nociceptin/orphanin FQ and cannabinoid systems in neuropathic mice. Pharmacol Biochem Behav. 2011;99:540–4. doi: 10.1016/j.pbb.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Gul H, YildIz O, Bilgin F, Guzeldemir ME. Cannabinoids blocks tactile allodynia in diabetic mice without attenuation of its anti-nociceptive effect. Neurosci Lett. 2004;368:82–6. doi: 10.1016/j.neulet.2004.06.060. [DOI] [PubMed] [Google Scholar]
- Ulugol A, Karadag HC, Ipci Y, Tamer M, Dokmeci I. The effect of WIN 55,212-2, a cannabinoid agonist, on tactile allodynia in diabetic rats. Neurosci Lett. 2004;371:167–70. doi: 10.1016/j.neulet.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abushaar M. Molecular Characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned Cdna. Nature. 1990;346:561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Seyrek M, Yalcin B, Ulugol A. Involvement of descending serotonergic and noradrenergic pathways in CB1 receptor-mediated anti-nociception. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:97–105. doi: 10.1016/j.pnpbp.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Seyrek M, Yalcin B, Ulugol A. Involvement of serotonergic system in cannabinoid analgesia. In: Van Bockstaele EJ, editor. Endocannabinoid regulation of monoamines in psychiatric and neurological disorders. New York: Springer; 2013. pp. 277–95. [Google Scholar]
- Guindon J, Beaulieu P, Hohmann AG. Pharmacology of the cannabinoid system. In: Beaulieu P, Lussier D, Porreca F, Dickenson AH, editors. Pharmacology of pain. Seattle: IASP Press; 2010. p. 6. [Google Scholar]
- Richardson JD. Cannabinoids modulate pain by multiple mechanisms of action. J Pain. 2000;1:2–14. [Google Scholar]
- Manzanares J, Julian MD, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4:239–57. doi: 10.2174/157015906778019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–3. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Gul H, Akar A, YildIz O, Bilgin F, Guzeldemir E. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11–6. doi: 10.1016/s0304-3959(03)00068-x. [DOI] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Vanderah TW, Makriyannis A, Porreca F. Inhibition of pain responses by activation of CB2 cannabinoid receptors. Chemistry and Physics of Lipids. 2002;121:191–200. doi: 10.1016/s0009-3084(02)00155-x. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–27. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Starowicz K, Di Marzo V. Non-psychotropic analgesic drugs from the endocannabinoid system: “Magic bullet” or “multiple-target” strategies? Eur J Pharmacol. 2013;716:41–53. doi: 10.1016/j.ejphar.2013.01.075. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Vandewater SA, Taffe MA. Cannabidiol attenuates deficits of visuospatial associative memory induced by Delta 9tetrahydrocannabinol. Brit J Pharmacol. 2013;170:1365–73. doi: 10.1111/bph.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre-Garriga J, Vila C, Clissold S, Montalban X. THC and CBD oromucosal spray (Sativex (R)) in the management of spasticity associated with multiple sclerosis. Expert Rev Neurother. 2011;11:627–37. doi: 10.1586/ern.11.47. [DOI] [PubMed] [Google Scholar]
- Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. Can Med Assoc J. 2008;178:1669–78. doi: 10.1503/cmaj.071178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth C, Mawani S, Brady S, Chan C, Liu CX, Mehina E, et al. An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain. 2012;153:2073–82. doi: 10.1016/j.pain.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Zogopoulos P, Vasileiou I, Patsouris E, Theocharis SE. The role of endocannabinoids in pain modulation. Fund Clin Pharmacol. 2013;27:64–80. doi: 10.1111/fcp.12008. [DOI] [PubMed] [Google Scholar]
- Cascio MG. PUFA-derived endocannabinoids: an overview. P Nutr Soc. 2013;72:451–9. doi: 10.1017/S0029665113003418. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2008;160:1–24. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- Fowler CJ. Anandamide uptake explained? Trends Pharmacol Sci. 2012;33:181–5. doi: 10.1016/j.tips.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Chapman V. Endocannabinoid metabolism and uptake: novel targets for neuropathic and inflammatory pain. Brit J Pharmacol. 2007;152:624–32. doi: 10.1038/sj.bjp.0707433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst M, Salim K, Burstein S, Conrad I, Hoy L, Schneider U. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. JAMA. 2003;290:1757–62. doi: 10.1001/jama.290.13.1757. [DOI] [PubMed] [Google Scholar]
- Pryce G, Visintin C, Ramagopalan SV, Al-Izki S, De Faveri LE, Nuamah RA, et al. Control of spasticity in a multiple sclerosis model using central nervous system-excluded CB1 cannabinoid receptor agonists. FASEB J. 2014;28:117–30. doi: 10.1096/fj.13-239442. [DOI] [PubMed] [Google Scholar]
- Kalliomaki J, Annas P, Huizar K, Clarke C, Zettergren A, Karlsten R, et al. Evaluation of the analgesic efficacy and psychoactive effects of AZD1940, a novel peripherally acting cannabinoid agonist, in human capsaicin-induced pain and hyperalgesia. Clin Exp Pharmacol P. 2013;40:212–8. doi: 10.1111/1440-1681.12051. [DOI] [PubMed] [Google Scholar]
- Ostenfeld T, Price J, Albanese M, Bullman J, Guillard F, Meyer I, et al. A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin J Pain. 2011;27:668–76. doi: 10.1097/AJP.0b013e318219799a. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Straiker A, Mackie K. CB2: therapeutic target-in-waiting. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:16–20. doi: 10.1016/j.pnpbp.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PA, Selley DE, Sim-Selley LJ, Welch SP. Low dose combination of morphine and [Delta]9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol. 2007;571:129–37. doi: 10.1016/j.ejphar.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Elevating endocannabinoid levels: pharmacological strategies and potential therapeutic applications. Proc Nutr Soc. 2014;73:96–105. doi: 10.1017/S0029665113003649. [DOI] [PubMed] [Google Scholar]
- Maione S, Costa B, Di Marzo V. Endocannabinoids: a unique opportunity to develop multitarget analgesics. Pain. 2013;154:S87–93. doi: 10.1016/j.pain.2013.03.023. [DOI] [PubMed] [Google Scholar].Piscitelli F, Di Marzo V. “Redundancy” of Endocannabinoid Inactivation: New Challenges and Opportunities for Pain Control. ACS Chem Neurosci. 2012;3:356–63. doi: 10.1021/cn300015x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Kinsey SG, Guo TL, Cravatt BF, Lichtman AH. Regulation of inflammatory pain by inhibition of fatty acid amide hydrolase. J Pharmacol Exp Ther. 2010;334:182–90. doi: 10.1124/jpet.109.164806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli A, Coccurello R, Rapino C, Di Serio S, Di Tommaso M, Vertechy M, et al. The novel reversible fatty acid amide hydrolase inhibitor ST4070 increases endocannabinoid brain levels and counteracts neuropathic pain in different animal models. J Pharmacol Exp Ther. 2012;342:188–95. doi: 10.1124/jpet.111.191403. [DOI] [PubMed] [Google Scholar]
- Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153:1837–46. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Naidu PS, Lichtman A, Onnis V. The case for the development of novel analgesic agents targeting both fatty acid amide hydrolase and either cyclooxygenase or TRPV1. Brit J Pharmacol. 2009;156:412–9. doi: 10.1111/j.1476-5381.2008.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morera E, De Petrocellis L, Morera L, Moriello AS, Ligresti A, Nalli M, et al. Synthesis and biological evaluation of piperazinyl carbamates and ureas as fatty acid amide hydrolase (FAAH) and transient receptor potential (TRP) channel dual ligands. Bioorg Med Chem Lett. 2009;19:6806–9. doi: 10.1016/j.bmcl.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Costa B, Bettoni I, Petrosino S, Comelli F, Giagnoni G, Di Marzo V. The dual fatty acid amide hydrolase/TRPV1 blocker, N-arachidonoylserotonin, relieves carrageenan-induced inflammation and hyperalgesia in mice. Pharmacol Res. 2010;61:537–46. doi: 10.1016/j.phrs.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–U111. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niphakis MJ, Johnson DS, Ballard TE, Stiff C, Cravatt BF. O-hydroxyacetamide carbamates as a highly potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chem Neurosci. 2012;3:418–26. doi: 10.1021/cn200089j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, et al. A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci. 2012;15:64–U82. doi: 10.1038/nn.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogestatt ED, Jonsson BAG, Ermund A, Andersson DA, Bjork H, Alexander JP, et al. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005;280:31405–12. doi: 10.1074/jbc.M501489200. [DOI] [PubMed] [Google Scholar]
- .Mallet C, Daulhac L, Bonnefont J, Ledent C, Etienne M, Chapuy E, et al. Endocannabinoid and serotonergic systems are needed for acetaminophen-induced analgesia. Pain. 2008;139:190–200. doi: 10.1016/j.pain.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Rogosch T, Sinning C, Podlewski A, Watzer B, Schlosburg J, Lichtman AH, et al. Novel bioactive metabolites of dipyrone (metamizol) Bioorg Med Chem. 2012;20:101–7. doi: 10.1016/j.bmc.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmas P, Ulugol A. Involvement of cannabinoid CB1 receptors in the antinociceptive effect of dipyrone. J Neural Transm. 2013;120:1533–8. doi: 10.1007/s00702-013-1052-7. [DOI] [PubMed] [Google Scholar]
- Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur J Pharmacol. 2006;531:280–1. doi: 10.1016/j.ejphar.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Escobar W, Ramirez K, Avila C, Limongi R, Vanegas H, Vazquez E. Metamizol, a non-opioid analgesic, acts via endocannabinoids in the PAG-RVM axis during inflammation in rats. Eur J Pain. 2012;16:676–89. doi: 10.1002/j.1532-2149.2011.00057.x. [DOI] [PubMed] [Google Scholar]
Disclaimer: This paper is for informational purposes only and does not constitute medical advice. Consult with a qualified healthcare professional before starting any new treatment, including cannabinoid therapy.