Abstract:
The endocannabinoid system (ECS) is a crucial signaling pathway involved in regulating a diverse range of physiological processes, including pain perception, mood, appetite, and immune function. Disruptions in ECS signaling have been implicated in various pathological conditions, leading to interest in potential therapeutic interventions leveraging the system. While modulating ECS activity through inhibitors or enhancers of endocannabinoid synthesis or degradation has shown promise, the use of phytocannabinoids derived from plants like Cannabis sativa as potential endocannabinoid replacements has gained considerable attention. This paper examines the evidence supporting and refuting the notion that phytocannabinoids can effectively act as endocannabinoid replacements, considering their mechanisms of action, bioavailability, receptor selectivity, and potential clinical applications and limitations.
1. Introduction:
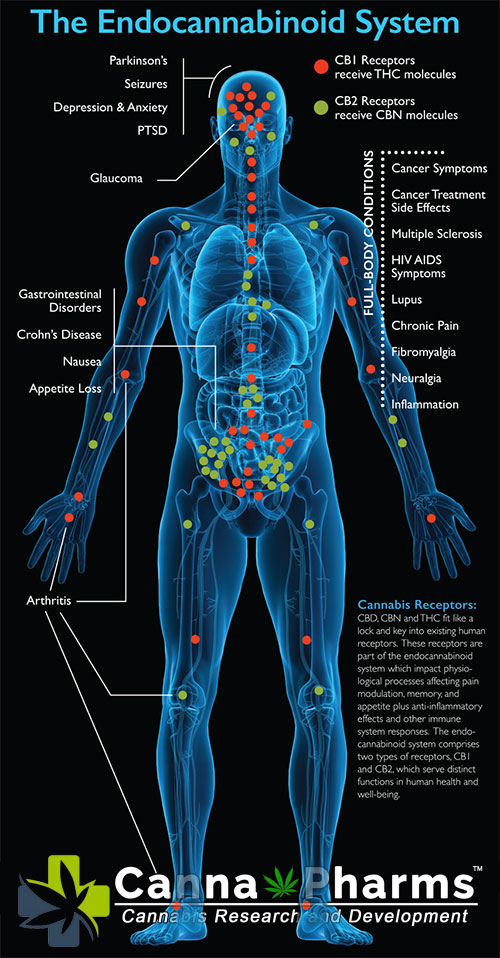
The ECS is a complex network comprising endogenous ligands (endocannabinoids like anandamide (AEA) and 2-arachidonoylglycerol (2-AG)), cannabinoid receptors (primarily CB1 and CB2 receptors), and enzymes responsible for the synthesis and degradation of endocannabinoids. Endocannabinoids are synthesized "on-demand" and act locally, contributing to a highly regulated and dynamic system. Dysregulation of the ECS, often termed "endocannabinoid deficiency," has been associated with conditions like migraine, fibromyalgia, and irritable bowel syndrome. This has spurred research into methods of modulating ECS function therapeutically.
Phytocannabinoids, naturally occurring compounds found in plants, particularly Cannabis sativa, interact with the ECS through various mechanisms. The most well-known phytocannabinoids, Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), exert diverse effects via direct and indirect interactions with cannabinoid receptors and other targets. This raises the question: can phytocannabinoids effectively replace or augment the function of endogenous endocannabinoids in situations of deficiency or dysregulation?
2. Mechanisms of Action: Differences and Similarities:
While both endocannabinoids and phytocannabinoids interact with the ECS, their mechanisms of action differ significantly.
-
Direct Receptor Binding: THC is a partial agonist at both CB1 and CB2 receptors, exhibiting a higher affinity for CB1 receptors, primarily located in the brain and central nervous system. This binding is responsible for the psychoactive effects of THC. AEA, on the other hand, is a partial agonist at CB1 receptors but with lower potency than THC. 2-AG is considered a full agonist at both CB1 and CB2 receptors, playing a crucial role in modulating synaptic transmission. CBD exhibits very weak affinity for both CB1 and CB2 receptors, suggesting that its therapeutic effects are mediated through other mechanisms.
-
Indirect Modulation of the ECS: CBD influences the ECS indirectly by modulating the levels of endocannabinoids. It inhibits the fatty acid amide hydrolase (FAAH) enzyme, which degrades AEA, leading to elevated AEA levels. It may also influence the transport of AEA and 2-AG. Endocannabinoids are synthesized "on-demand" and rapidly degraded, ensuring a transient and localized effect.
-
Off-Target Effects: Phytocannabinoids, particularly CBD, interact with a variety of other targets, including transient receptor potential (TRP) channels, G-protein coupled receptors (GPCRs), and serotonin receptors. These off-target effects contribute to their diverse pharmacological profile and may be responsible for some of their therapeutic benefits. Endocannabinoids, while having some off-target effects, primarily act through the ECS.
3. Bioavailability and Pharmacokinetics:
The bioavailability and pharmacokinetics of phytocannabinoids and endocannabinoids differ considerably.
-
Endocannabinoids: Endocannabinoids are synthesized locally and rapidly degraded, demonstrating a short half-life and limited systemic exposure.
-
Phytocannabinoids: The bioavailability of phytocannabinoids, especially when administered orally, is often low due to first-pass metabolism in the liver. Factors like formulation, route of administration, and individual metabolism significantly affect their absorption and distribution. Furthermore, THC can accumulate in adipose tissue, leading to prolonged effects.
These differences in pharmacokinetics and bioavailability raise concerns about the ability of phytocannabinoids to precisely mimic the spatially and temporally controlled signaling of endocannabinoids.
4. Receptor Selectivity and Specificity:
Endocannabinoids are synthesized and degraded locally, allowing for precise control over receptor activation and signal duration. In contrast, systemic administration of phytocannabinoids can result in widespread receptor activation, potentially leading to undesired side effects.
-
CB1 Receptor Mediated Effects: The psychoactive effects associated with THC can be problematic for some patients, limiting its usefulness as a direct endocannabinoid replacement, particularly in individuals susceptible to anxiety or psychosis.
-
CB2 Receptor Activation: While CB2 receptors are primarily expressed on immune cells, their modulation by both endocannabinoids and phytocannabinoids can have therapeutic implications for inflammatory and autoimmune diseases. However, systemic activation of CB2 receptors by phytocannabinoids may not precisely replicate the context-dependent signaling mediated by endocannabinoids.
5. Clinical Applications and Limitations:
Phytocannabinoids have shown potential in treating various conditions, including chronic pain, epilepsy, and nausea. However, their use as endocannabinoid replacements also presents limitations.
-
Pain Management: THC's analgesic properties, mediated by CB1 receptor activation, have been used in pain management. However, the potential for tolerance, dependence, and cognitive impairment limits its long-term use. CBD's analgesic effects, potentially mediated through TRP channels and indirect modulation of the ECS, may offer a safer alternative for some patients.
-
Epilepsy: CBD has demonstrated efficacy in treating certain forms of epilepsy, particularly Dravet syndrome and Lennox-Gastaut syndrome. This highlights the potential of phytocannabinoids to address specific endocannabinoid system-related pathologies, although the specific mechanisms of action remain under investigation.
-
Neuroprotection: Both endocannabinoids and phytocannabinoids have shown neuroprotective properties in preclinical studies. However, translating these findings to clinical applications requires further research, considering the complexities of the ECS and the potential for off-target effects.
6. Considerations for Endocannabinoid Deficiency Syndromes:
The concept of "clinical endocannabinoid deficiency (CECD)" has emerged to describe conditions where ECS dysfunction contributes to pathological symptoms. While supporting research is still developing, there's growing interest in using cannabinoid therapies to address these conditions.
-
Tailoring Therapies: A key approach to treating CECD is to tailor cannabinoid therapies to the specific needs of each patient. This includes considering the individual's genetic makeup, the specific condition being treated, and the potential for drug interactions.
-
Low-Dose Approach: Some clinicians advocate for a low-dose approach to cannabinoid therapy, aiming to gently stimulate the ECS without overwhelming it. This may reduce the risk of side effects and tolerance.
-
Lifestyle Factors: Optimizing ECS function may also involve lifestyle modifications, such as diet, exercise, and stress management, which can influence endocannabinoid levels and receptor sensitivity.
7. Conclusion:
Phytocannabinoids cannot be considered direct replacements for endocannabinoids due to significant differences in their mechanisms of action, pharmacokinetics, receptor selectivity, and specific context of action. While THC directly activates cannabinoid receptors, potentially mimicking some endocannabinoid effects, it also carries the risk of psychoactive side effects and tolerance. CBD modulates the ECS indirectly and engages with various other signaling pathways, exhibiting a more complex pharmacological profile.
However, phytocannabinoids hold promise for modulating the ECS therapeutically in specific contexts. Their potential to address endocannabinoid dysfunction and alleviate symptoms in various conditions warrants further investigation. Future research should focus on:
-
Identifying the precise mechanisms of action of phytocannabinoids.
-
Developing targeted therapies that selectively modulate the ECS without causing undesired side effects.
-
Identifying biomarkers for endocannabinoid deficiency to better diagnose and treat ECS-related disorders.
-
Conducting rigorous clinical trials to evaluate the efficacy and safety of phytocannabinoids in treating specific conditions.
Ultimately, a comprehensive understanding of the ECS and the complex interactions of phytocannabinoids is essential for harnessing their therapeutic potential and developing effective strategies for treating ECS-related disorders. The goal is not necessarily to "replace" endocannabinoids, but to strategically modulate the ECS to restore balance and improve patient outcomes.