The Lag in Legitimacy: Why Cannabinoid Therapy Remains on the Margins for ALS Treatment
Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig's disease, is a devastating neurodegenerative disorder characterized by the progressive loss of motor neurons, leading to muscle weakness, paralysis, and ultimately, respiratory failure. Despite decades of research, the only FDA-approved therapies for ALS provide modest benefits in extending lifespan, highlighting the urgent need for novel and effective treatments. Cannabinoids, a diverse group of compounds derived from the Cannabis sativa plant, have shown promise in preclinical studies for neuroprotection, anti-inflammation, and symptom management. However, despite anecdotal evidence and burgeoning public interest, cannabinoid therapy has yet to achieve widespread acceptance and legitimacy as a standard treatment for ALS. This paper will explore the multifaceted reasons behind this lag, encompassing scientific limitations, regulatory hurdles, societal stigma, and economic disincentives.
1. Limited and Inconsistent Scientific Evidence:
A primary obstacle to the acceptance of cannabinoid therapy for ALS is the scarcity of robust, large-scale clinical trials demonstrating its efficacy and safety. While preclinical studies, often conducted in vitro or in animal models, have shown promising neuroprotective effects of cannabinoids, these findings have not consistently translated to human studies.
- Varied Preclinical Findings: Studies using cell cultures and animal models of ALS have reported that cannabinoids like THC and CBD can mitigate oxidative stress, reduce inflammation, and protect motor neurons from excitotoxicity, key pathological processes in ALS. However, the specific cannabinoids, dosages, and delivery methods employed vary significantly, leading to inconsistent outcomes and difficulty in translating these findings to human trials.
- Small Sample Sizes and Methodological Limitations in Clinical Trials: Existing clinical trials evaluating cannabinoids for ALS have been plagued by small sample sizes, lack of placebo control groups, variations in cannabinoid formulations, and ill-defined outcome measures. These methodological limitations make it difficult to draw definitive conclusions regarding efficacy and safety. Furthermore, the subjective nature of some symptom assessments (e.g., pain, spasticity) makes it challenging to objectively evaluate the therapeutic benefits of cannabinoids.
- Heterogeneity of ALS: ALS is a clinically heterogeneous disease, with varying symptom presentations, rates of progression, and underlying genetic factors. This heterogeneity complicates the design of clinical trials, as it can be difficult to identify patient subgroups who may be more likely to benefit from cannabinoid therapy.
2. Regulatory Hurdles and Classification of Cannabis:
The legal status of cannabis and cannabinoids remains a significant barrier to research and clinical application. In many countries, including the United States, cannabis is still classified as a Schedule I controlled substance, severely restricting its availability for research purposes.
- Restricted Access to Research-Grade Cannabinoids: The stringent regulations surrounding the cultivation, possession, and use of cannabis make it difficult for researchers to obtain high-quality, standardized cannabinoid products for clinical trials. This limits the number and scope of studies that can be conducted.
- Conflicting Federal and State Laws: In countries where cannabis is legal at the state level but remains illegal federally, researchers face legal uncertainty and potential conflicts with federal funding agencies. This presents a significant disincentive for conducting research on cannabinoids.
- Approval Pathways for Cannabinoid-Based Pharmaceuticals: The lack of clear regulatory pathways for approving cannabinoid-based pharmaceuticals creates uncertainty for pharmaceutical companies and investors. The cost and complexity of navigating the regulatory approval process can be prohibitive, particularly for smaller companies.
3. Societal Stigma and Misinformation:
The historical stigma associated with cannabis use continues to influence public perception and acceptance of cannabinoid therapy, hindering its adoption as a legitimate treatment for ALS.
- Negative Perceptions of Cannabis: Decades of negative propaganda and misinformation surrounding cannabis have created deeply ingrained biases that can make it difficult for patients, healthcare providers, and policymakers to objectively evaluate the potential benefits of cannabinoid therapy.
- Self-Medication and Unregulated Products: Due to the lack of FDA-approved cannabinoid therapies for ALS, many patients turn to self-medication with unregulated cannabis products, often obtained from dispensaries or online sources. This poses several risks, including inconsistent product quality, inaccurate labeling, potential for interactions with other medications, and lack of medical supervision.
- Resistance from the Medical Community: Some physicians remain hesitant to recommend or prescribe cannabinoid therapy due to concerns about its safety, efficacy, and potential for drug interactions. This resistance can be attributed to a lack of knowledge about cannabinoids, concerns about legal liability, and lingering stigma associated with cannabis use.
4. Economic Disincentives and Lack of Industry Investment:
The uncertain regulatory landscape, coupled with the high cost of clinical research and product development, creates economic disincentives for pharmaceutical companies to invest in cannabinoid therapy for ALS.
- Limited Patent Protection: The natural origin of cannabis and cannabinoids makes it difficult to obtain strong patent protection for cannabinoid-based therapies. This reduces the potential for exclusivity and profitability, discouraging investment from pharmaceutical companies.
- High Cost of Clinical Trials: Conducting large-scale, placebo-controlled clinical trials is expensive and time-consuming. The uncertain regulatory environment and limited patent protection make it difficult for companies to justify the significant investment required to bring a cannabinoid-based therapy for ALS to market.
- Focus on More Lucrative Markets: Pharmaceutical companies often prioritize research and development efforts on more common and profitable diseases, leaving rare and orphan diseases like ALS underfunded.
5. The Need for Integrative Research and Patient-Centered Approaches:
Overcoming these obstacles requires a multifaceted approach that prioritizes rigorous scientific research, addresses regulatory hurdles, combats societal stigma, and fosters patient-centered care.
- Prioritize Well-Designed Clinical Trials: Future clinical trials should employ robust methodologies, including large sample sizes, placebo control groups, standardized cannabinoid formulations, and well-defined outcome measures. Stratification of patients based on genetic factors and disease progression may help identify subgroups who are more likely to benefit from cannabinoid therapy.
- Harmonize Regulatory Frameworks: Governments should harmonize regulatory frameworks to facilitate research on cannabinoids and streamline the approval process for cannabinoid-based pharmaceuticals. This includes rescheduling cannabis at the federal level and establishing clear guidelines for the cultivation, testing, and distribution of cannabinoid products.
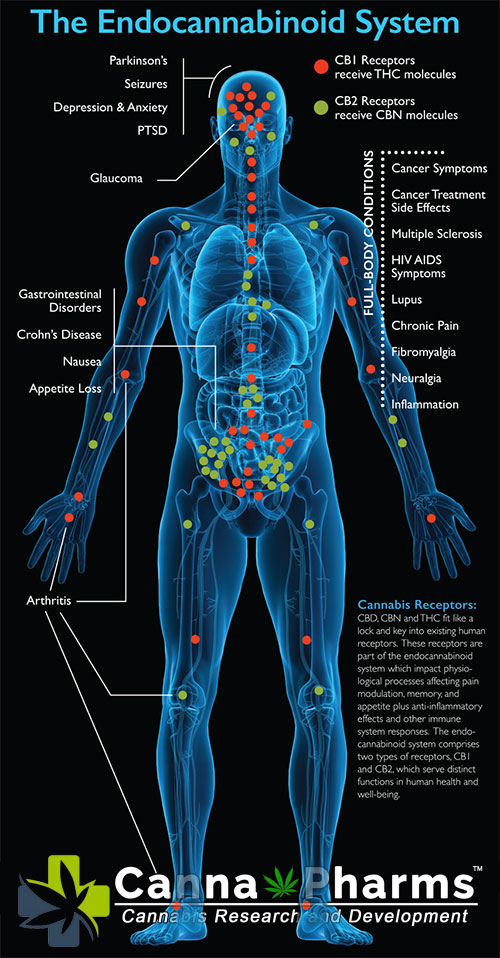
- Promote Education and Awareness: Public education campaigns are needed to dispel misinformation about cannabis and promote evidence-based understanding of the potential benefits and risks of cannabinoid therapy. Healthcare providers should receive comprehensive training on the endocannabinoid system, the pharmacology of cannabinoids, and the clinical applications of cannabinoid therapy.
- Incorporate Patient Perspectives: Patient input should be incorporated into the design of clinical trials and the development of treatment guidelines. Understanding patient preferences and priorities will help ensure that cannabinoid therapies are tailored to meet the specific needs of individuals living with ALS.
Conclusion:
While anecdotal evidence and preclinical studies suggest potential benefits of cannabinoid therapy for managing symptoms and potentially slowing disease progression in ALS, significant challenges remain that hinder its widespread adoption as a legitimate treatment. These challenges include limited and inconsistent scientific evidence, regulatory hurdles, societal stigma, and economic disincentives. Addressing these challenges requires a concerted effort from researchers, policymakers, healthcare providers, and patient advocacy groups to prioritize rigorous scientific research, harmonize regulatory frameworks, promote education and awareness, and incorporate patient perspectives. Only through a comprehensive and collaborative approach can we unlock the full potential of cannabinoid therapy and improve the lives of individuals living with ALS. Future research should focus on identifying specific cannabinoids or cannabinoid combinations that are most effective for specific ALS subtypes, elucidating the mechanisms of action of cannabinoids in ALS, and developing safe and effective delivery methods. Ultimately, the path to legitimacy for cannabinoid therapy in ALS hinges on the generation of robust clinical evidence that demonstrates its efficacy and safety, paving the way for its integration into standard treatment protocols.