Abstract:
Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Lobar Degeneration (FTLD) are devastating neurodegenerative diseases characterized by the mislocalization and aggregation of TDP-43. Genetic mutations in the ATXN2 gene, encoding ataxin-2, are recognized risk factors for both these diseases. Ataxin-2 interacts with TDP-43, and its dysfunction, particularly in the context of expanded polyglutamine repeats, can exacerbate TDP-43 pathology. This paper explores the potential of cannabinoid therapy as a novel approach to modulate ATXN2 mutation-induced TDP-43 misfolding. We will discuss the interplay between ATXN2, TDP-43, and the endocannabinoid system, highlighting potential therapeutic mechanisms and future research directions.
1. Introduction:
Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Lobar Degeneration (FTLD) are progressive neurodegenerative disorders that share significant pathological overlap, including the accumulation of misfolded and aggregated TDP-43 (TAR DNA-binding protein 43) in affected neurons and glial cells. TDP-43 is a DNA/RNA-binding protein involved in various cellular processes, including RNA splicing, transport, and regulation. Aberrant processing and cytoplasmic mislocalization of TDP-43 are considered key drivers of neurodegeneration in ALS and FTLD.
Genetic factors play a significant role in the pathogenesis of these diseases. Mutations in the ATXN2 gene, encoding ataxin-2, have been identified as a common genetic risk factor for both ALS and FTLD. Ataxin-2 is a cytoplasmic protein involved in RNA processing and stress granule formation. Expanded polyglutamine (polyQ) repeats within the ATXN2 gene are associated with increased risk of disease, with longer repeats correlating with earlier disease onset.
While the precise mechanisms by which mutant ATXN2 contributes to TDP-43 pathology remain under investigation, evidence suggests that dysfunctional ataxin-2 disrupts normal TDP-43 processing and localization. The endocannabinoid system (ECS), a ubiquitous signaling network involved in a wide range of physiological functions, has shown promise in mitigating neuroinflammation and neurodegeneration. This paper hypothesizes that cannabinoid therapy, by modulating ECS activity, could offer a therapeutic strategy to counteract the effects of ATXN2 mutations on TDP-43 misfolding and aggregation.
2. The Interplay of ATXN2 and TDP-43:
Ataxin-2 interacts directly with TDP-43, influencing its localization and processing. Studies have shown that:
- ATXN2 facilitates TDP-43 mRNA metabolism: Normal ataxin-2 plays a role in regulating the stability and translation of TDP-43 mRNA. Disruption of this regulation by mutant ataxin-2 could lead to dysregulation of TDP-43 protein levels.
- ATXN2 influences stress granule formation: Both TDP-43 and ataxin-2 are integral components of stress granules, transient cytoplasmic aggregates formed under cellular stress. Mutant ataxin-2 is thought to promote the aberrant aggregation of TDP-43 within these stress granules, potentially seeding the formation of insoluble TDP-43 aggregates.
- ATXN2 mutations exacerbate TDP-43 pathology: Animal models expressing mutant ATXN2 demonstrate increased TDP-43 mislocalization and aggregation compared to controls. This suggests that mutant ataxin-2 directly contributes to TDP-43-related neurodegeneration.
The precise molecular mechanisms underlying this interplay are complex and likely involve alterations in RNA binding, protein-protein interactions, and cellular stress responses. Unraveling these mechanisms is crucial for developing targeted therapeutic interventions.
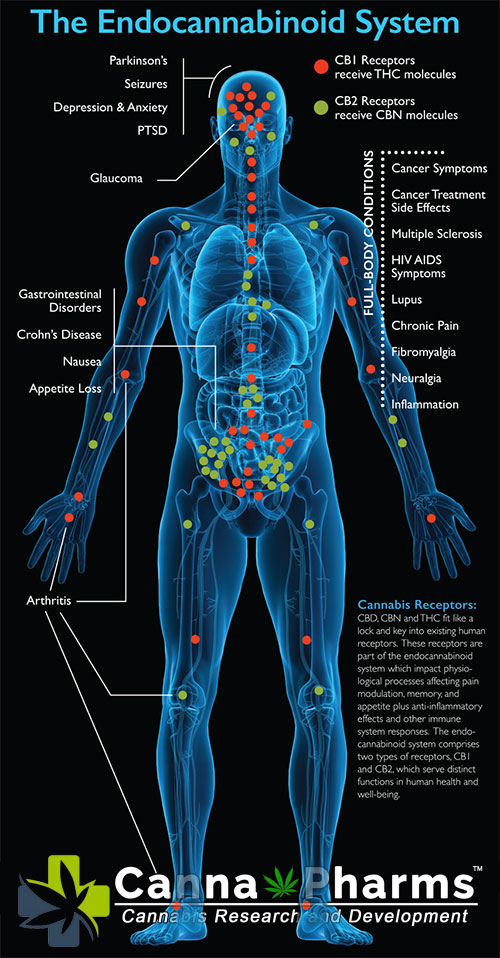
3. The Endocannabinoid System and Neuroprotection:
The endocannabinoid system (ECS) is a complex lipid signaling network composed of cannabinoid receptors (CB1 and CB2), endocannabinoids (e.g., anandamide and 2-arachidonoylglycerol), and enzymes responsible for their synthesis and degradation. The ECS plays a critical role in maintaining homeostasis within the central nervous system (CNS). Its activation has demonstrated neuroprotective effects in various neurodegenerative disease models, including ALS and FTLD.
Key mechanisms by which the ECS exerts its neuroprotective effects include:
- Neuroinflammation Reduction: Activation of CB2 receptors, primarily located on microglia and other immune cells within the brain, suppresses the release of pro-inflammatory cytokines and chemokines, mitigating neuroinflammation.
- Antioxidant Effects: Cannabinoids can act as antioxidants, scavenging free radicals and reducing oxidative stress, a significant contributor to neuronal damage in neurodegenerative diseases.
- Modulation of Glutamate Excitotoxicity: The ECS can modulate glutamate neurotransmission, potentially reducing excitotoxicity, a process implicated in neuronal death in ALS.
- Autophagy Enhancement: Certain cannabinoids have been shown to promote autophagy, a cellular process responsible for clearing damaged proteins and organelles, including TDP-43 aggregates.
4. Cannabinoid Therapy as a Potential Modulator of ATXN2-Mediated TDP-43 Misfolding:
Based on the interplay between ATXN2 and TDP-43, and the neuroprotective properties of the ECS, we hypothesize that cannabinoid therapy can modulate ATXN2 mutation-induced TDP-43 misfolding through several potential mechanisms:
- Attenuation of Stress Granule Formation: Cannabinoid activation of CB receptors could potentially modulate stress granule dynamics, preventing the aberrant aggregation of TDP-43 within these granules, particularly in the context of mutant ATXN2.
- Regulation of TDP-43 mRNA Metabolism: The ECS is known to influence gene expression. Cannabinoids may influence the expression of genes involved in TDP-43 mRNA processing, potentially counteracting the disruptive effects of mutant ATXN2.
- Promotion of TDP-43 Degradation: By enhancing autophagy, cannabinoids may promote the clearance of misfolded and aggregated TDP-43, mitigating the accumulation of pathological TDP-43 in cells expressing mutant ATXN2.
- Reduction of Neuroinflammation: The anti-inflammatory effects of CB2 receptor activation could protect vulnerable neurons from further damage induced by neuroinflammation exacerbated by the presence of misfolded TDP-43 and mutant ATXN2.
5. Challenges and Future Directions:
While the potential of cannabinoid therapy in modulating ATXN2-mediated TDP-43 misfolding is promising, significant challenges remain:
- Specificity: Targeting the ECS requires careful consideration of receptor selectivity and downstream effects to avoid potential side effects. Developing cannabinoids that selectively activate CB2 receptors or target specific intracellular signaling pathways may be crucial.
- Delivery: Effective delivery of cannabinoids to the CNS is essential. Strategies such as nanotechnology-based drug delivery systems and blood-brain barrier penetration enhancers may be necessary to optimize therapeutic efficacy.
- Translatability: Preclinical studies utilizing cell culture models and animal models expressing mutant ATXN2 and exhibiting TDP-43 pathology are essential to validate the therapeutic potential of cannabinoid therapy and to optimize dosage and treatment regimens.
- Clinical Trials: Ultimately, randomized controlled clinical trials will be necessary to assess the safety and efficacy of cannabinoid therapy in patients with ALS and FTLD carrying ATXN2 mutations.
Future research directions should focus on:
- Investigating the precise molecular mechanisms by which cannabinoids influence ATXN2-TDP-43 interactions.
- Developing and testing novel cannabinoid-based therapeutics with improved receptor selectivity and bioavailability.
- Conducting preclinical studies using relevant animal models to evaluate the efficacy of cannabinoid therapy in reducing TDP-43 pathology and improving functional outcomes.
- Exploring personalized medicine approaches, considering genetic background (ATXN2 polyQ length) and individual responses to cannabinoid therapy.
6. Conclusion:
ATXN2 mutations represent a significant risk factor for ALS and FTLD, contributing to TDP-43 misfolding and neurodegeneration. Cannabinoid therapy, by modulating the endocannabinoid system, offers a potential therapeutic strategy to counteract the effects of mutant ATXN2 on TDP-43 pathology. While significant research is needed to elucidate the precise mechanisms and optimize therapeutic efficacy, the potential of cannabinoid therapy as a novel treatment option for ALS and FTLD warrants further investigation. This hypothesis provides a framework for future research aimed at developing targeted and effective therapies for these devastating neurodegenerative diseases.