Abstract: Amyotrophic Lateral Sclerosis (ALS) is a devastating neurodegenerative disease characterized by the progressive loss of motor neurons. While the etiology of ALS remains complex and multifactorial, accumulating evidence highlights the involvement of neuroinflammation in disease pathogenesis. Cannabinoids, acting primarily through CB2 receptors expressed on immune cells, possess immunomodulatory properties, including the ability to downregulate cytokine and chemokine production. This review explores the rationale for investigating cannabinoids as potential therapeutic agents in ALS, focusing on their ability to modulate neuroinflammation and potentially slow disease progression.
Introduction:
Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig's disease, is a fatal neurological disorder affecting motor neurons in the brain and spinal cord. This progressive loss of motor neurons leads to muscle weakness, atrophy, and ultimately, paralysis and death. While genetic factors contribute to a subset of ALS cases, the majority are sporadic, with the underlying causes remaining largely unknown. However, a growing body of research implicates neuroinflammation as a key contributor to the pathogenesis of both familial and sporadic ALS.
The Role of Neuroinflammation in ALS:
Neuroinflammation, characterized by the activation of glial cells (microglia and astrocytes) and the release of pro-inflammatory cytokines and chemokines, has been consistently observed in the central nervous system (CNS) of ALS patients and animal models. Microglia, the resident immune cells of the brain, become activated in response to neuronal damage and release cytotoxic factors, contributing to further neuronal injury. Astrocytes, another type of glial cell, also become reactive and can contribute to inflammation and excitotoxicity, further exacerbating neuronal dysfunction. Elevated levels of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, and chemokines like CCL2 and CXCL10, have been detected in the cerebrospinal fluid (CSF) and spinal cord of ALS patients, suggesting a sustained inflammatory environment that contributes to motor neuron degeneration (Citation 53, Citation 54). This chronic inflammatory state is not a mere bystander effect, but rather a significant driver of disease progression.
The Cannabinoid System and Immune Modulation:
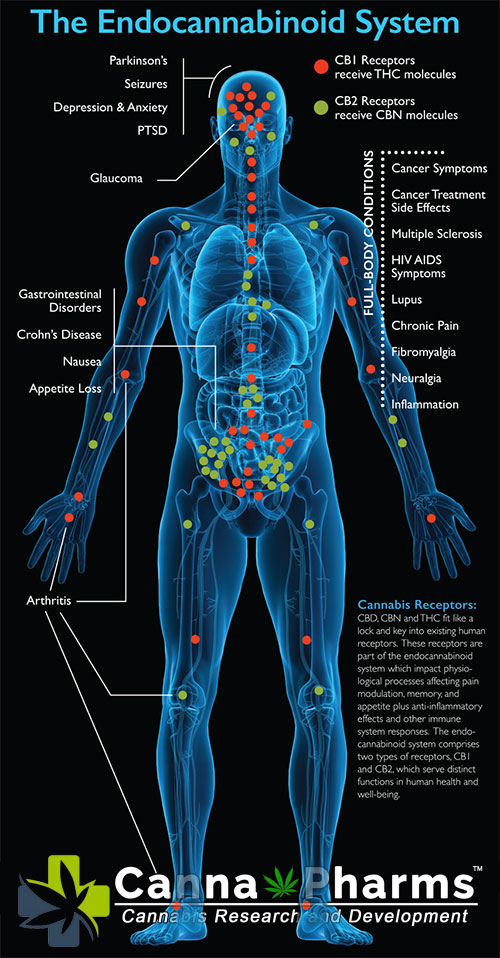
The endocannabinoid system (ECS) is a complex neuromodulatory system involved in a wide range of physiological processes, including pain regulation, mood, appetite, and immune modulation. The ECS consists of endogenous cannabinoids (endocannabinoids), cannabinoid receptors (CB1 and CB2), and enzymes responsible for their synthesis and degradation.
CB1 receptors are primarily located in the CNS and are responsible for the psychoactive effects associated with cannabinoids. In contrast, CB2 receptors are predominantly expressed on immune cells, including microglia, macrophages, and lymphocytes. The discovery of CB2 receptors on immune cells has opened the door to understanding the role of cannabinoids in regulating the immune system.
Cannabinoids acting on CB2 receptors can modulate immune responses by downregulating the production of pro-inflammatory cytokines and chemokines. This downregulation occurs through various mechanisms, including:
Inhibition of NF-κB signaling: NF-κB is a key transcription factor that regulates the expression of pro-inflammatory genes. Activation of CB2 receptors can inhibit NF-κB signaling, leading to reduced cytokine and chemokine production (Citation 49).
Modulation of MAPK pathways: Mitogen-activated protein kinases (MAPKs) are signaling pathways involved in regulating cell growth, differentiation, and apoptosis. CB2 receptor activation can modulate MAPK pathways, contributing to the suppression of inflammatory responses (Citation 50).
Induction of apoptosis in activated immune cells: Cannabinoids can induce apoptosis in activated microglia and macrophages, reducing the overall inflammatory burden in the CNS (Citation 51).
Promotion of regulatory T cell (Treg) development: Tregs are a subset of T cells that suppress immune responses. Some studies suggest that cannabinoids may promote the development and function of Tregs, leading to immune tolerance and reduced inflammation (Citation 52).
Cannabinoids as a Potential Therapeutic Strategy in ALS:
Given the evidence supporting the role of neuroinflammation in ALS pathogenesis and the ability of cannabinoids to modulate immune responses via CB2 receptors, cannabinoids represent a potentially promising therapeutic avenue for ALS. By targeting the inflammatory cascade, cannabinoids could potentially slow disease progression and improve patient outcomes.
Potential Benefits of Cannabinoid Therapy in ALS:
Reduction of Microglial Activation: Cannabinoids can inhibit the activation of microglia, reducing the release of cytotoxic factors and inflammatory mediators.
Attenuation of Astrogliosis: Cannabinoids may also reduce the reactivity of astrocytes, diminishing their contribution to inflammation and excitotoxicity.
Downregulation of Pro-inflammatory Cytokine Production: By inhibiting the production of pro-inflammatory cytokines such as TNF-α and IL-1β, cannabinoids can reduce the overall inflammatory burden in the CNS.
Neuroprotection: Some studies suggest that cannabinoids may have direct neuroprotective effects, protecting motor neurons from damage and promoting their survival.
Symptom Management: Beyond immunomodulation, cannabinoids may also provide symptomatic relief for ALS patients by reducing pain, spasticity, and anxiety.
Challenges and Future Directions:
While the rationale for using cannabinoids in ALS is compelling, several challenges need to be addressed:
Limited Clinical Data: To date, there are limited clinical trials evaluating the efficacy and safety of cannabinoids in ALS. Further clinical studies are needed to determine the optimal dose, route of administration, and specific cannabinoid formulations for ALS patients.
Psychoactive Effects: The psychoactive effects associated with certain cannabinoids, particularly THC, can be a concern for some patients. Developing non-psychoactive cannabinoid formulations or selectively targeting CB2 receptors could mitigate these side effects.
Complexity of the ECS: The ECS is a complex system with intricate interactions. Further research is needed to fully understand the mechanisms by which cannabinoids modulate neuroinflammation in ALS and to identify specific targets within the ECS for therapeutic intervention.
Individual Variability: Responses to cannabinoid therapy can vary between individuals due to factors such as genetics, disease stage, and concurrent medications. Personalized cannabinoid-based therapies may be necessary to optimize treatment outcomes.
Conclusion:
Neuroinflammation plays a significant role in the pathogenesis of ALS, contributing to motor neuron degeneration and disease progression. Cannabinoids, by virtue of their immunomodulatory properties and ability to downregulate inflammatory responses, hold promise as potential therapeutic agents for ALS. Further research, particularly well-designed clinical trials, is crucial to fully evaluate the efficacy and safety of cannabinoid therapy in ALS and to optimize its potential for improving patient outcomes. While the journey to finding effective treatments for ALS is ongoing, the potential of cannabinoids to modulate neuroinflammation provides a compelling area of investigation that warrants continued exploration.